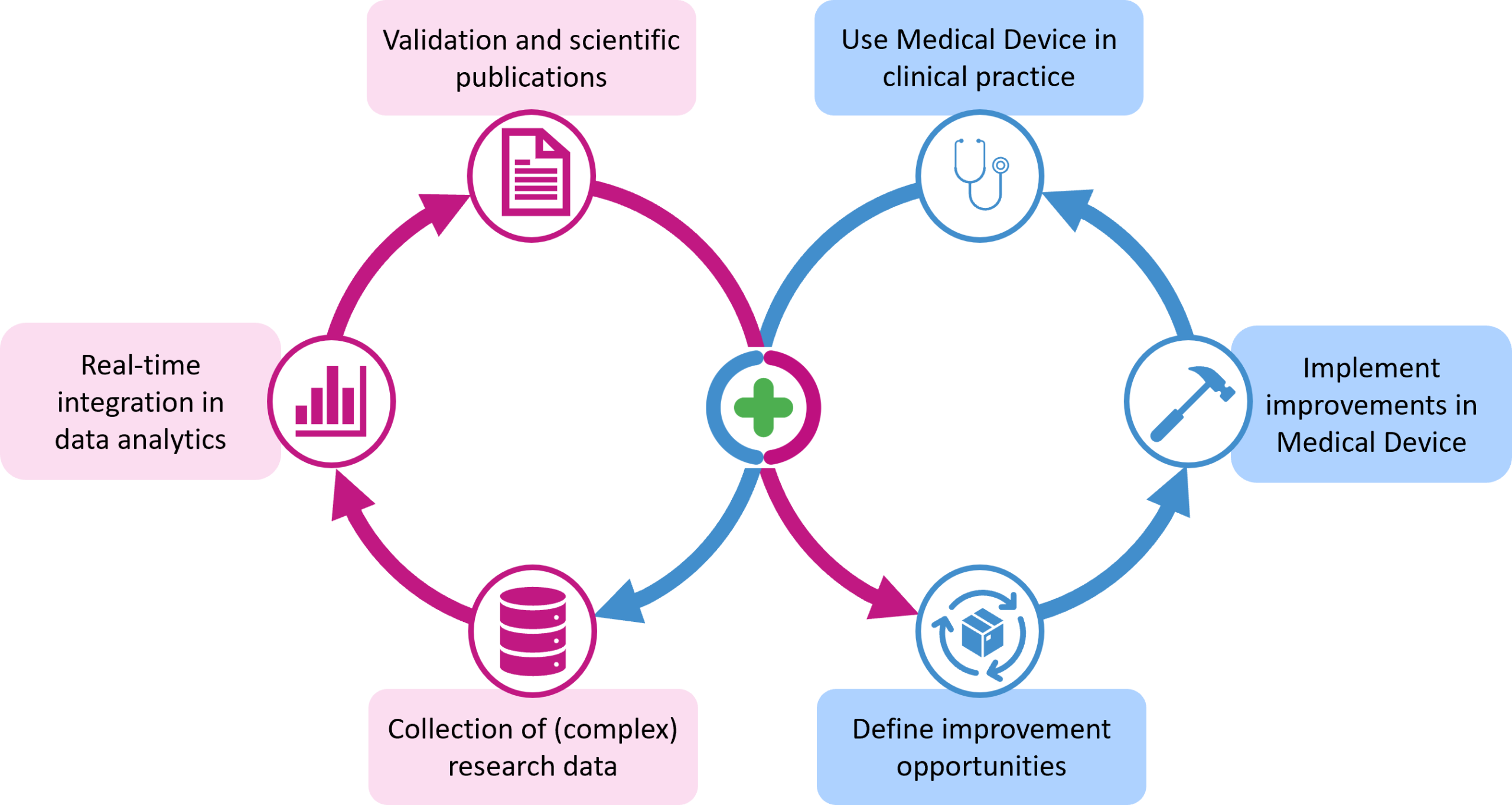
The data collected from the application of a Clinical Decision Support System and stored securely in LogiqSuite’s MDM platform allows scientists to enrich the data with additional patient outcomes and to analyze new insights and potentially improve or recalibrate the prediction model implemented in the medical device.
Medical devices may be updated based on the latest scientific insights and advancements. This includes incorporating new or updated prediction models and adapting to changes in clinical guidelines. These updates ensure the CDSS provides accurate and reliable results that align with ongoing scientific advancements and recommendations, keeping devices at the forefront of medical practice.
In the EU, the development and maintenance of medical device software are governed by regulations such as the Medical Device Regulation (MDR 2017/745) and standards like ISO 13485 (Quality Management Systems for Medical Devices) and IEC 62304 (Software Lifecycle Processes). These require manufacturers to systematically monitor, review, and update their devices – supporting continual improvement.
3.1 Risk management
Continual improvement involves the periodic review of risk management files (ISO 14971). New risks identified from post-market surveillance data can be mitigated through software updates or process improvements.
3.2 Post-Market Surveillance
Under EU MDR, manufacturers must engage in ongoing post-market surveillance. This includes collecting user feedback, reporting incidents, and implementing corrective/preventive actions – all feeding into continual improvement cycles.Collecting the data of a medical device implemented in clinical practice constitutes additional evidence on the performance of the device, extremelyvaluable for the post-market clinical follow-up evaluation.
Medical device software such as CDSS is often updated to correct defects, address cybersecurity risks, and enhance technical and functional features. Each update goes through assessment, validation, and regulatory review, ensuring continuous compliance and improvement.We ensure rigorous documentation and traceability of all changes, maintaining integrity and transparency throughout the software lifecycle.