LogiqSuiteis a medical data management platform consisting of three interoperableand configurable cloud-based systems: LogiqCare, LogiqScience and LogiqAnalytics. Both LogiqCare and LogiqSciencefacilitate structured data collection -distinguishing between identifiable patients and pseudonymoussubjects-allowing seamlesscollaborationbetween care and research.In LogiqAnalytics, the platform provides real-time and de-identified data to empower advanced analytics, enabling efficient availability of healthcare data for medical data science. A scientific paper describing the use of LogiqSuite in various use cases of precision medicine has been published.
Healthcare professionalsbenefit from standard functionalities such as patient case management and test requests. LogiqCare empowers care professionals to meticulously record patient data for diagnosis and treatment while analyzing results. Furthermore, the platform integrates third-party applications and Clinical Decision Support Systems, enhancing diagnostic and patient management processes.
“LogiqSuite is a medical data management system that can serve as a valuable tool to evaluate data for both research and clinical care.Our goal is to differentially analyze the complex data to determine which parameters are associated with outcomes for different patient subsets. This way, we can improve and further personalize prognosis, diagnosis, and treatment.”
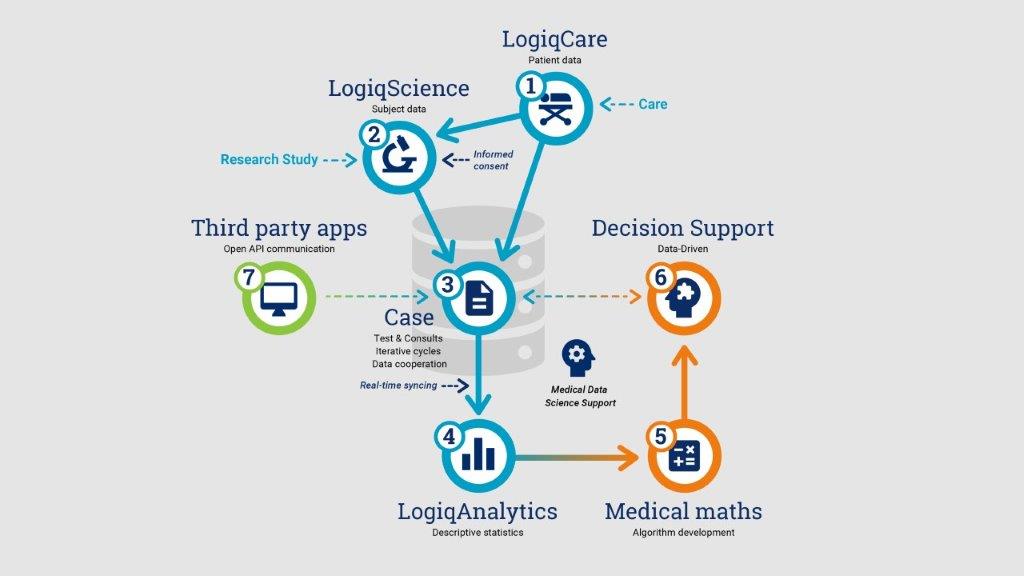
1.LogiqCarefocuses on patient data management within a healthcare setting, utilizing the Patient-Case structure. It supports the real-time collection and structured storage of clinical care data, facilitating collaboration among healthcare providers from various institutions.
2.LogiqScienceis designed for research, accommodating the entry of clinical and laboratory data using pseudonymous subjects. It functions as an electronic case-report form (eCRF) for registries, cohorts, and clinical studies. Additionally, it is empowered with electronic patient reported outcome (ePRO) functionality.LogiqScience offers the ability to set-up a custom data model and import and standardize existingdatasets for advanced analysis, as required.
3.Case:within LogiqSuite, a Case represents a focal point for data collection, often related to a patient’s disease or study visit. It contains all related inputs from healthcare providers, lab tests, patient feedback (ePRO), and/or device data.The case also facilitates collaboration between physicians from different medical disciplines or organizations, both in care and research projects.
4.LogiqAnalyticsis a separate SQL database consisting of de-identified and privacy-unenriched data, synced in real-time from LogiqCare andLogiqScience. It facilitates medical data science, descriptive statistics and AI-research. We offer seamless integration with fully customizable PowerBI reports, for monitoring and evaluation of key-performance indicators. Robust access control on the LogiqAnalyticsdatabase, empowers secure collaboration through controlled sharing of data subsets when required.
5.Medical mathdeals with the development, application, and integration of new mathematical solutionsinto medicine, such as results from research using LogiqAnalytics. These methods includemedical insightscombined withsurvival analysiswith competing risks (Fine-Gray),imputationfor missing data in survival analysis,machine learningfor parameter selection in survival analysis, and explainable artificial intelligence (XAI) of clinical decision support like diagnostic predictions.
6.Clinical Decision Support Systems(CDSS)implement validated prediction modelsasSoftware-as-a-Medical Device (SaMD)to facilitate personalized medicine in clinical practice. For example U-Prevent, for risk management and prevention of (cardio)vascular diseases. Our CDSS solutions are built according toMDRcompliant processes and, if applicable, MDR-certified. A CDSS solution can be integrated intoLogiqSuite, to prefill with available clinical data and save real-word outcomes. By combining both platforms, wedrive healthcare innovation through Continual learning and improvement
- Third Party apps:LogiqSuite’sopen API allows seamless integration with third-party solutions, includingmachine-2-machine communication, import of external data sources, clinical decision support systems, as well as wearables or other devices that collect personal health data.
“To the researcher, LogiqScience offers high-quality data for clinical studies. Healthcare professionals can use various standard functionalities, such as test requests and subject-case management. In addition, they can use connected solutions (including third-party solutions), such as for Clinical Decision Support.”
LogiqSuiteconsists of LogiqCare, LogiqScienceandLogiqAnalytics as medical data management systems, compliant with strict privacy and security regulations regarding managing patient data and certified for clinical data registration according toISO27001, NEN7510, 7512, 7513. The LogiqSuite platform is hosted in Microsoft Azure in the European Economic Area (EEA) with GDPR and medical data constraints. Data manipulations are recorded in audit trails, user activities are monitored and logged, and data entries can be signed off.
Casesare completed with data from Consultations (e.g. direct observations or questionaries sent to patients) and two- or three-layered Test cascades for structured follow-up (e.g. for analysis performed at another department). The data templates are customizable and include conditional logic, input validation rules, and calculations to accommodate the requirements of a specific application.
Access control is regulated by roles (e.g. viewer, editor, administrator) and attributes (e.g. department, science, care).
Data modelsare defined and validated by key users or data managers and implemented and maintained by ORTEC Logiqcare. LogiqSuite is a multilingual application, allowing users and patients to work in their preferred language.
With LogiqSuite we strive for FAIR(Findable, Accessible, Interoperable & Reusable)data. Each parameter could be connected to multiple data dictionaries to communicate with the different (inter)national standards of data communication. Theopen APIallows data exchange. Combined with professional data stewardship, this allows for organizing a sustainable data cohort to be used over a long period of time. Real-time syncing of datato LogiqAnalytics enables the possibility of Continual improvement of healthcare and clinical decision support systems.







